Hydrogen sulfide can be formed in the following reaction: H2(g) + 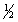 S2(g)
S2(g)  H2S(g) H°rxn = -92 kJ
H2S(g) H°rxn = -92 kJ
The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
Definitions:
Cross Elasticity
Cross elasticity of demand quantifies how the demand for a good or service is affected by the price change of another related good, reflecting their substitutability or complementarity.
Complements
Goods or services that are used together, where the use of one increases the demand for the other.
Substitutes
Goods or services that can be used in place of each other; as the price of one increases, the demand for the other increases.
Price Inelastic
Describes a situation where the demand for a product does not significantly change with a change in its price.
Q3: A concentration cell consists of two Zn/Zn<sup>2+</sup>
Q9: Given that each 3-base sequence in DNA
Q19: Identify the principal organic product when benzyl
Q19: A 0.15 M solution of chloroacetic acid
Q43: Which of the following is true for
Q50: A reactant R is being consumed in
Q62: A certain process has <font face="symbol"></font>S<sub>univ</sub> >
Q64: At 500°C the equilibrium constant, K<sub>p </sub>,
Q82: Select the correct name for the following
Q114: Predict the products for the following set