The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. 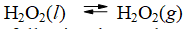
Use the following thermodynamic information at 298 K to determine this temperature. 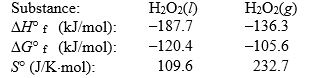
Definitions:
Magnitude
The size, extent, or importance of something.
Correlation Coefficient
A numerical indicator that shows the degree to which multiple variables co-vary.
Negative Life Events
Significant adverse events or situations that can lead to stress and have potentially lasting impacts on an individual's mental health.
Perceptions of Stress
How an individual views or interprets stress, which can significantly affect their psychological and physical response to it.
Q5: When the following redox equation is balanced
Q8: Which one of the following descriptions relating
Q31: Once a reaction system reaches equilibrium, the
Q32: Which of the following ligands is most
Q35: A student adds 0.1 mol of oxalic
Q42: A solution is prepared by adding 4.50
Q44: The equilibrium constant K<sub>c</sub> for the reaction
Q56: Use the following information to calculate the
Q96: All strong acids have weak conjugate bases.
Q116: Citric acid has an acid dissociation constant