Multiple Choice
Iron(III) oxide can be reduced by carbon monoxide. 
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature. 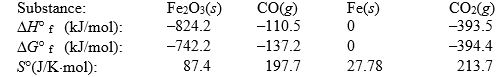
Recognize the role and significance of whistle-blowers in maintaining organizational ethics and integrity.
Understand the concept and implications of whistle-blowing in organizations.
Grasp the philosophies underpinning moral capital and idealism in business ethics.
Recognize the impact of ethical decisions on environmental conservation and consumer safety.
Definitions:
Related Questions
Q8: For a given reaction, a change in
Q24: The r-process occurs during supernova explosions.
Q25: Why is it necessary to flush peripheral
Q36: A system delivers 225 J of heat
Q38: Calculate the solubility of zinc hydroxide, Zn(OH)<sub>2</sub>,
Q41: For all processes, both q and <font
Q45: Electrons are produced at the cathode of
Q57: Hydrogen iodide, HI, is formed in an
Q64: The redox reaction of peroxydisulfate with iodide
Q93: The following reaction is at equilibrium at