Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 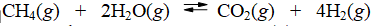
Definitions:
Critical Incident
An event or situation that significantly affects the operation of a system or organization, often used in performance management and analysis.
False Negative
A test result which incorrectly indicates that a particular condition or attribute is absent.
Contrast Errors
In performance assessment, these are errors made when comparing one individual's performance too closely against others, rather than against objective criteria, leading to exaggerated differences.
Halo Errors
Halo errors occur in performance appraisal when the evaluator's overall impression of an employee, either positive or negative, influences judgments about specific performance traits.
Q21: Ammonia will react with oxygen in the
Q39: A popular buffer solution consists of carbonate
Q39: Aluminum and magnesium form are light metals
Q41: Ammonia is synthesized in the Haber process:<br>N<sub>2</sub>(g)
Q44: Which of the following transition elements can
Q54: Consider the figure which shows <font face="symbol"></font>G°
Q56: How many unpaired electrons are there in
Q58: A salt bridge provides a path for
Q63: A catalyst accelerates a reaction because<br>A) it
Q68: In the shorthand notation for cells, a