The formation constant for the reaction 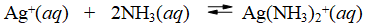
Is Kf = 1.7 × 107 at 25°C. What is G° at this temperature?
Definitions:
ATP
Adenosine triphosphate, a molecule that provides energy for many processes in living cells by being converted to ADP (adenosine diphosphate).
NADPH
Nicotinamide adenine dinucleotide phosphate, a coenzyme involved in anabolic reactions, such as lipid and nucleic acid synthesis, acting as a reducing agent.
Autotrophs
Organisms capable of producing their own food through photosynthesis or chemosynthesis, utilizing sunlight or chemical reactions.
Heterotrophs
Organisms that obtain their energy by consuming other living or deceased organisms, as they cannot synthesize their own food.
Q2: Which one of the following is a
Q21: You are given pure samples of pentane,
Q26: Gamma rays are not deflected by an
Q39: Chlorine atoms act as heterogeneous catalysts in
Q47: Which one of the following equations correctly
Q59: How many grams of oxygen gas will
Q77: The conversion of the chromate ion (CrO<sub>4</sub><sup>2-</sup>)
Q82: The equilibrium constant, K<sub>p </sub>, for the
Q83: Hydrofluoric acid (HF) has a K<sub>a</sub> value
Q89: Write the ion product expression for magnesium