The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. 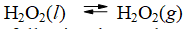
Use the following thermodynamic information at 298 K to determine this temperature. 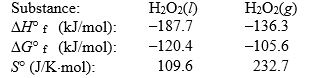
Definitions:
Scatter Plot
A graphical representation used to display values for typically two variables for a set of data, showing how much one variable is affected by another.
Widely Dispersed
Spread out over a large area or among a large number of people; not concentrated in one place.
Confidence Intervals
An interval of numbers, calculated from statistics of a sample, assumed to include the value of a not yet identified parameter of a population.
Level Of Confidence
A measure of belief in one's abilities or the certainty of an event happening.
Q24: In order to write the correct mass-action
Q35: Tetrahedral complexes can exhibit both optical and
Q36: Which of the following will be paramagnetic?<br>A)
Q40: A system which undergoes an adiabatic change
Q62: For the reaction 3A(g) + 2B(g) <font
Q63: A catalyst accelerates a reaction because<br>A) it
Q82: It is believed that two carbon-12 nuclei
Q87: In the compound [Ni(en)<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub>]SO<sub>4</sub> (where en =
Q89: Explain how a disulfide bridge can arise
Q109: Formic acid is a monoprotic acid with