Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 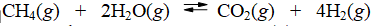
Definitions:
Application Letter
A written document submitted to an employer by a job applicant, detailing the applicant's credentials and interest in the position.
Screening
The process of evaluating or examining something to filter out unwanted or unsuitable elements.
Interview Process
A series of steps including preparation, questioning, and evaluation, used by employers to assess potential candidates for a job.
Selection
The process of choosing from a number of options or individuals.
Q4: A concentration cell is based on the
Q9: A reaction has an activation energy of
Q34: You need to use KH<sub>2</sub>PO<sub>4</sub> and K<sub>2</sub>HPO<sub>4</sub>
Q43: A reaction is second-order with respect to
Q48: Natural gas, or methane, is an important
Q51: Which of the following aqueous mixtures would
Q55: a. Write a balanced equation for the
Q71: Which of the following is not a
Q76: Select the nuclide that completes the following
Q88: Which of the following transition elements can