The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K. 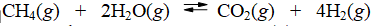
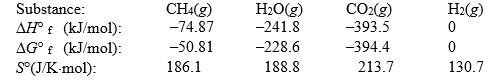
Definitions:
Collateral
Property or assets pledged by a borrower to secure a loan, subject to seizure on default.
Third-Party Beneficiary
A person who is not a party to a contract but who has the right to enforce it because the parties to the contract made the contract with the intent to benefit him.
Implied
Something not explicitly stated but understood to be included or involved through indirect indications or logical inference.
Statute of Frauds
A legal concept that requires certain types of contracts to be in writing and signed by the party to be charged, in order to be enforceable.
Q3: Which of the following affects the activation
Q3: The substance HClO<sub>4 </sub>is considered<br>A) a weak
Q9: What will be the effect of adding
Q9: What is the mechanism by which control
Q12: Two cells are connected in series, so
Q25: After 4 half-lives, the fraction of a
Q26: Gamma rays are not deflected by an
Q27: A 55-kg person exposed to thorium-234 receives
Q46: A voltaic cell consists of a Hg/Hg<sub>2</sub><sup>2+</sup>
Q53: Examine the following half-reactions and select the