Consider the reaction of iodine with manganese dioxide 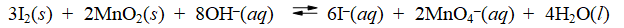
The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate G° for the reaction at 25°C.
Definitions:
Researcher(s)
Individuals or groups that conduct systematic investigations to establish facts or principles, typically in an academic or professional context.
Servant Leader
A leadership philosophy where the main goal of the leader is to serve others, prioritizing the needs of the team or organization over their own.
Career Advancement
The process of making progress in one's job or profession, often involving gaining additionalskills, responsibilities, and higher positions.
Servant Approach
A leadership philosophy centered on serving others first, focusing on the growth and well-being of team members and the communities to which they belong.
Q14: When the following redox equation is balanced
Q24: In order to write the correct mass-action
Q26: A certain transition element has the stable
Q31: In one or two short sentences each,
Q47: Why is the +2 oxidation state so
Q57: Fill in missing sub- and superscripts for
Q67: The solubility of silver chromate is 0.0287
Q67: Examine the following half-reactions and select the
Q69: Write the mass-action expression, Q<sub>c </sub>, for
Q90: Consider the equilibrium reaction shown below. B<sub>2</sub>(g)