Consider the reaction of iodine with manganese dioxide 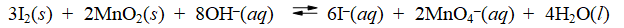
The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate E°cell for the reaction at 25°C.
Definitions:
Substance P
A neuropeptide involved in the transmission of pain and other sensory signals in the central and peripheral nervous system.
Serotonin
A neurotransmitter that plays a crucial role in mood regulation, digestion, sleep, and various other bodily functions.
Dopamine
A neurotransmitter involved in reward, motivation, and various other neurological and physiological functions.
Semicircular Canals
Three fluid-filled tubes located in the inner ear that play a critical role in maintaining balance and spatial orientation by detecting rotational movements.
Q1: Write a complete, balanced equation to represent
Q2: A system that does no work but
Q4: The process that selectively extracts a metal
Q11: The greater the energy of activation, E<sub>a</sub>,
Q18: A phosphate buffer (H<sub>2</sub>PO<sub>4</sub><sup>-</sup>/HPO<sub>4</sub><sup>2-</sup>) has a pH
Q32: Gamma rays are high energy electrons.
Q42: The reaction<br>2NaOH(aq) + H<sub>2</sub>SO<sub>4</sub>(aq) <font face="symbol"></font> Na<sub>2</sub>SO<sub>4</sub>(aq)
Q44: In order for a process to be
Q69: Use the following data to calculate the
Q92: A buffer is prepared by adding 1.00