The isotopes of promethium, 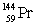 and
and 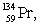 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
Definitions:
Man-Made Resources
Resources created by humans through processing or altering natural materials, which are used to produce goods and services.
Production Process
The method and sequence of operations involved in the production of a good or service.
Demand for Products
The total amount of a product or service that consumers are willing and able to purchase at various prices during a specified period.
Resource Dependency
A theory highlighting how the external resources that organizations need to survive and thrive affect their behavior and strategies.
Q1: Sterile gloves are required when:<br>A) collecting blood
Q5: The solubility of magnesium phosphate is 2.27
Q49: A current of 1000. A flows for
Q50: Your favorite candy bar, Gummy Beakers, contains
Q55: The main effect of the biosphere on
Q57: Samples that require chilling immediately after collection
Q61: Chain of custody refers to the:<br>A) method
Q73: What is the E°<sub>cell</sub> for the cell
Q75: Select the nuclide that completes the following
Q76: Which of the following atoms has the