Refer to the table below. 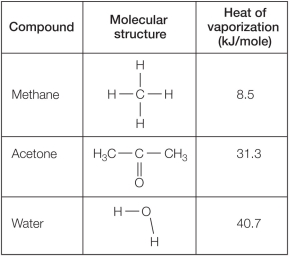 Which statement explains reasons for differences in the heat of vaporization for these compounds?
Which statement explains reasons for differences in the heat of vaporization for these compounds?
Definitions:
Proteins
Proteins are large, complex molecules made up of amino acids and are essential for the structure, function, and regulation of the body's tissues and organs.
Organic Soup
A hypothetical mixture of organic molecules from which life on Earth could have originated, prebiotically existing in the Earth's early oceans.
DNA Or RNA
Molecules that contain the genetic information for the synthesis of proteins and control of cellular activities; DNA is double-stranded while RNA is typically single-stranded.
Enzymes
Biological catalysts that speed up the rate of chemical reactions in the body without being consumed or permanently altered.
Q16: The nurse researcher is considering whether the
Q17: In response to low blood sugar, the
Q21: Which is required for natural selection to
Q21: In 1972, Stanley Miller filled test tubes
Q39: Meteorites from space<br>A) carried cyanobacteria that provided
Q149: Refer to the figures below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q158: The information needed to produce proteins is
Q162: Triglycerides are composed of<br>A) glycerol and fatty
Q223: RNA molecules that act as catalysts are
Q225: Which is an example of science?<br>A) An