Refer to the figures below. 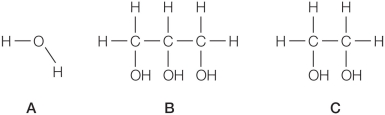 Rank the compounds in order of lowest to highest heat capacity per mole of compound.
Rank the compounds in order of lowest to highest heat capacity per mole of compound.
Definitions:
Anions
Negatively charged ions that are formed when an atom gains one or more electrons, commonly found in various chemical reactions and solutions.
Electrons
Electrons are subatomic particles with a negative charge, found in all atoms, and play a key role in chemical reactions and electricity.
Positive Charge
An electrical charge with more protons than electrons, commonly found in cations or positive ions.
Negative Charge
A negative charge refers to the electric charge carried by particles with more electrons than protons, often resulting in an attractive force toward positively charged particles.
Q8: Molecules that both attract and repel water
Q67: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q115: If the pH of an acid rain
Q125: If a protein is placed in urea,
Q127: Which statement about fatty acids is true?<br>A)
Q131: A cell<br>A) always contains a nucleus.<br>B) is
Q135: Water is a polar molecule.This property contributes
Q188: An atom with 20 protons and 25
Q229: When an egg is exposed to extreme
Q230: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"