Refer to the table below. 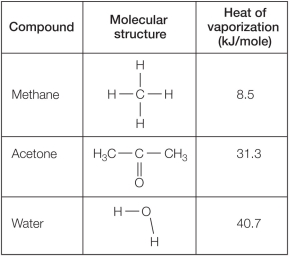 Which statement explains reasons for differences in the heat of vaporization for these compounds?
Which statement explains reasons for differences in the heat of vaporization for these compounds?
Definitions:
Family Therapist
A psychotherapist specializing in treating family issues and dynamics through family systems theory and therapy approaches.
Family Members
Individuals who are related by blood, marriage, or adoption, often forming a basic social unit called a family.
Humanistic Psychotherapists
Psychotherapists who focus on individual potential and stress the importance of growth and self-actualization.
Self-Concept
Self-Concept is an individual's perception of themselves, encompassing beliefs, feelings, and thoughts about one's abilities, appearance, and personality.
Q4: Much of what we know about the
Q8: The nurse is creating a community education
Q16: The nurse researcher is considering whether the
Q20: The nurse has accepted a position as
Q61: Refer to the figure below, showing the
Q141: Because of the similarities shared by many
Q195: Aspartate and glutamate can form hydrogen bonds
Q204: Refer to the image below, which shows
Q206: Which statement concerning chemical reactions and catalysts
Q234: Which description applies to polysaccharides, polypeptides, and