Refer to the table below. 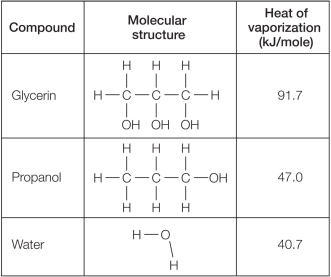 Which statement explains reasons for differences in the heat of vaporization for these compounds?
Which statement explains reasons for differences in the heat of vaporization for these compounds?
Definitions:
Accountability
The obligation of an individual or organization to account for its activities, accept responsibility for them, and disclose the results in a transparent manner.
Appreciative Inquiry
An approach to organization change that focuses on what is positive by asking participants to share their experiences and successes, examine the challenges, and explore what is working well.
Organization Change
The process through which a company or organization undergoes a transition to achieve a desired outcome, often involving restructuring, strategy transformation, or cultural change.
Planned Change
An intended, purposive attempt to make something different.
Q12: The nurse is offering free occult blood
Q13: All of the following are examples of
Q14: Refer to the figure showing one of
Q23: Oil and water do not mix easily
Q30: Suppose that you are working in a
Q64: Refer to the reaction shown. C<sub>3</sub>H<sub>8</sub> +
Q113: The use of energy from sunlight to
Q147: Scientists interested in human biology typically perform
Q216: Suppose you want to construct a protein
Q221: A molecule contains five atoms and has