Refer to the figure below. 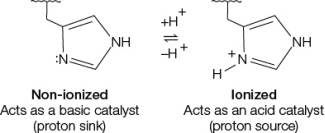 The figure shows how a histidine group at the active site of an enzyme could function either as a base (left) or as an acid (right) .The pKa of histidine is 6.0, which means that at pH 6.0, there are equal numbers of ionized and nonionized histidine groups since a pKa is an acid dissociation constant.With this in mind, what would you expect about the pH sensitivity of enzyme X that uses histidine as a base catalyst compared with enzyme Y that uses histidine as an acid catalyst?
The figure shows how a histidine group at the active site of an enzyme could function either as a base (left) or as an acid (right) .The pKa of histidine is 6.0, which means that at pH 6.0, there are equal numbers of ionized and nonionized histidine groups since a pKa is an acid dissociation constant.With this in mind, what would you expect about the pH sensitivity of enzyme X that uses histidine as a base catalyst compared with enzyme Y that uses histidine as an acid catalyst?
Definitions:
Q14: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q33: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q80: Suppose the reaction A <font face="symbol"></font> B
Q83: Denatured enzymes are<br>A) ribozymes.<br>B) synthesized in vitro.<br>C)
Q91: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q104: Which statement about NADPH is false?<br>A) It
Q130: The conversion of malate to oxaloacetate in
Q176: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q183: Refer to the figure below, which shows
Q240: Refer to the table below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"