Refer to the figure below. 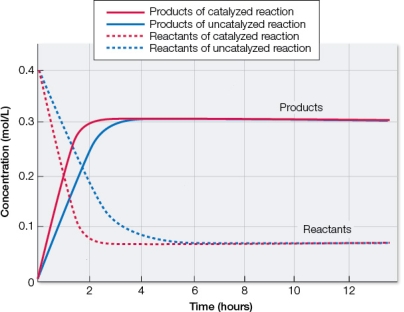 Based on the graph, which statement best describes these chemical reactions?
Based on the graph, which statement best describes these chemical reactions?
Definitions:
Lock Up
To confine someone or something securely, often used in the context of imprisonment.
New Recruits
Individuals who have newly joined an organization, institution, or the armed forces, often undergoing training and orientation.
Rehabilitation
The process of restoring someone to health or normal life through training and therapy after incarceration, addiction, or illness.
Law-abiding
Adhering to and respecting established laws and regulations.
Q17: Which sequence lists the various mechanisms cells
Q68: Which is a similarity between gap junctions
Q77: Different organisms have different ATP yields from
Q78: What part of the electromagnetic spectrum has
Q91: Refer to the graphs below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q120: Refer to the figure below. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5650/.jpg"
Q149: Which of the following represents kinetic energy?<br>A)
Q157: How many six-carbon sugar molecules are produced
Q213: If stomata on a plant leaf close,
Q228: In an experiment, the F<sub>1</sub> portion of