The figure shows the pV diagram for a certain thermodynamic process. In this process, 1500 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 9.00 × 104 Pa. If the volume of the system increases from 0.020 m3 to 0.050 m3, calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative. 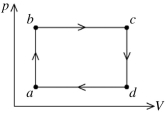
Definitions:
Securely Attached
A term used in attachment theory describing individuals who have healthy emotional bonds and relationships, often resulting from consistent and responsive caregiving in early life.
Mutual Regulation
Process by which infant and caregiver communicate emotional states to each other and respond appropriately.
Strange Situation
A research method used to evaluate a child's pattern of attachment to their caregivers, involving observations of children's reactions to separations and reunions.
Disorganized-Disoriented
Refers to a type of attachment behavior in children characterized by a lack of clear strategy to manage their emotions and interactions with caregivers.
Q4: The exterior of a supersonic airplane is
Q16: A mass M is attached to an
Q18: A long straight very thin wire on
Q22: If a current of 2.4 A is
Q23: A parallel-plate capacitor consists of two parallel,
Q27: An alpha particle is moving at a
Q30: A proton beam that carries a total
Q32: A nonuniform, 80.0-g, meterstick balances when the
Q49: The second law of thermodynamics leads us
Q57: At 50.0°C, the average translational kinetic energy