What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol∙K) . 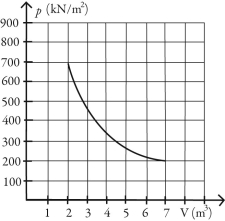
Definitions:
Economic Profit
The difference between total revenue and total costs, including both explicit and implicit costs, representing surplus or loss.
Investment Return
The gain or loss on an investment over a specified period, expressed as a percentage of the investment's cost.
Interest Rate
The percentage charged by lenders to borrowers for the use of money, typically expressed at an annual rate.
Economic Profit
The difference between total revenues earned by a firm and its total opportunity costs.
Q4: The weight of spaceman Speff at the
Q13: An initially-stationary electric dipole of dipole moment
Q16: A charged capacitor is connected to an
Q19: If the electric potential in a region
Q21: What is the root-mean-square value of the
Q22: What is the ratio of the escape
Q26: A half-ring (semicircle) of uniformly distributed charge
Q38: The reason an astronaut in an earth
Q49: The second law of thermodynamics leads us
Q51: A horizontal wire carries a current straight