What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol∙K) . 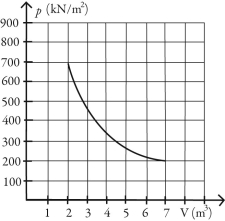
Definitions:
Misrepresentation
A misrepresentation of truth by one individual to another that leads to the latter being persuaded into agreeing to a contract.
Insurer
A company or entity which provides insurance coverage to individuals or organizations against potential losses or damages.
Defenses
Legal strategies and arguments used in court to negate, justify, or excuse alleged criminal or civil liabilities.
Judgment Pro Rata
A legal principle where the amount awarded or decided in judgment is distributed proportionally among creditors or claimants based on the size of their claims.
Q1: In the figure, two solenoids are side
Q4: Four resistors are connected across an 8-V
Q23: A vertical wire carries a current vertically
Q27: An alpha particle is moving at a
Q28: Consider the circuit shown in the figure.
Q34: A planet has two small satellites in
Q40: An air-filled capacitor stores a potential energy
Q44: A monatomic ideal gas undergoes an isothermal
Q47: A large cylindrical water tank is mounted
Q53: You want to insert an aluminum rod,