An electron inside a hydrogen atom is confined to within a space of 0.110 nm. What is the minimum uncertainty in the electron's velocity? ( 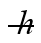 = 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
= 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
Definitions:
Four-Wheel Drive
A vehicle drive system where power is distributed to all four wheels simultaneously, enhancing traction.
Liable in Tort
Legal responsibility arising from a civil case in which harm has been caused to an individual or property.
Intoxicated Person
An individual whose normal capacity to act or make decisions is significantly impaired due to the consumption of alcohol or drugs.
Mentally Ill
A broad term covering a range of mental health conditions that affect mood, thinking, and behavior.
Q1: According to many studies of death anxiety
Q5: The dying trajectory<br>A) of communicable diseases is
Q12: The magnitude of the electric field at
Q13: A series ac circuit consists of a
Q14: A 15-turn rectangular loop of wire of
Q19: The figure shows two long, parallel current-carrying
Q30: The long straight wire in the figure
Q33: A set of five possible wave functions
Q34: A double-convex thin lens is made of
Q46: If the diameter of a radar dish