The excited state of a certain atom is 3.2 eV ± 0.21 eV. ( 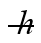 = 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
(a) What is the average lifetime of this state?
(b) If the excited energy were doubled to 6.4 eV ± 0.21 eV, how would the lifetime be affected?
Definitions:
Demographic Statistics
Numerical data relating to the characteristics of a population, such as age, gender, income, and education, used for market segmentation and analysis.
Quantified
Refers to the process of expressing or measuring something in terms of quantity or numbers, making it possible to analyze and compare objectively.
Customer Value Chain
A conceptual framework that outlines the series of steps a company takes to create value for the customer, from product development through to after-sales service.
Cross-Impact Matrix
A tool used to analyze and visualize the effects of one variable on another within a certain context, aiding in decision-making and scenario planning.
Q8: A ray of light consisting of blue
Q12: A wire segment 1.2 m long carries
Q19: An electron is in an infinite square
Q20: A spaceship approaches the earth with a
Q21: An excited <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="An excited
Q23: A certain isotope has a half-life of
Q24: Which of the following is/are important in
Q25: You want to confine an electron in
Q26: Light of wavelength 425.0 nm in air
Q46: The magnitude of the Poynting vector of