The excited state of a certain atom is 3.2 eV ± 0.21 eV. ( 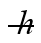 = 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s = 6.591 × 10-16 eV ∙ s, 1 eV = 1.60 × 10-19 J)
(a) What is the average lifetime of this state?
(b) If the excited energy were doubled to 6.4 eV ± 0.21 eV, how would the lifetime be affected?
Definitions:
Q2: The vibrational frequency of an HF molecule
Q3: If a nucleus decays by β<sup>+</sup> decay
Q8: A ray of light consisting of blue
Q12: According to Kastenbaum, a "death system"<br>A) includes
Q15: A person who enrolls in a course
Q17: What is the greatest total angular momentum
Q22: An electron with kinetic energy 2.80 eV
Q24: The stability of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="The stability
Q42: The material used in certain nuclear bombs
Q81: A lens is made with a focal