The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 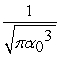 e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Definitions:
Environmental Management
The process of overseeing and controlling the impact of human activities on the natural environment to ensure sustainability and conservation.
Uniform Approach
A Uniform Approach implies a standardized method or strategy adopted across an organization to ensure consistency and coherence in achieving its objectives.
Q5: African-American attitudes toward death show that they
Q8: The voltage across the dees of a
Q12: If the accuracy in measuring the velocity
Q25: You want to confine an electron in
Q29: Most deaths in contemporary American society occur<br>A)
Q33: The analysis in Chapter 3 of four
Q34: In a laboratory accident a work area
Q36: An archaeologist finds the <sup>14</sup>C in a
Q40: A double-concave lens has equal radii of
Q50: Three spaceships A, B, and C are