The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r) = 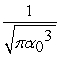 e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius, which is equal to 5.29 × 10-11 m. What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
Definitions:
Variable Overhead
Costs that vary with the level of production output, such as utilities for manufacturing equipment, which are not fixed.
Standard Cost
An estimated or pre-determined cost of manufacturing a product or performing a service, used for budgeting and performance evaluation.
Variable Manufacturing Overhead
Indirect production costs that fluctuate with the level of production output, such as utilities for the manufacturing plant.
Direct Labor-Hours
The total number of hours worked by employees directly involved in manufacturing goods or providing services.
Q2: Certain planes of a crystal of halite
Q8: A diatomic has a moment of inertia
Q9: Light consisting of a mixture of red
Q14: A series LRC circuit consists of a
Q17: What is the greatest total angular momentum
Q21: An electron is in an infinite square
Q27: A set of twins, Andrea and Courtney,
Q42: The material used in certain nuclear bombs
Q66: An optical system comprises in turn, from
Q81: A lens is made with a focal