A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state. What is the frequency of vibration of the molecule? (h = 6.626 × 10-34 J ∙ s, 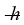 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
Definitions:
Surfactant
A substance that reduces the surface tension of a liquid, aiding in processes such as lung function and cleaning.
Alveoli
Tiny air sacs in the lungs where gas exchange takes place, oxygen enters the blood, and carbon dioxide is removed.
Bicarbonate Ions
The bicarbonate ion (HCO3-) plays a crucial role in maintaining the body's pH balance, particularly in the buffering of blood plasma.
Carbaminohemoglobin
Hemoglobin carrying carbon dioxide.
Q1: A camera used for aerial surveillance has
Q3: Light from a monochromatic source shines through
Q4: A simple refracting telescope provides large magnification
Q7: American Indians and Native Alaskans often view
Q15: The vignette in Chapter 5 describes a
Q15: If a galaxy is receding from us
Q15: What is the frequency of the light
Q31: The only INVALID electron state and shell
Q37: An ultraviolet source produces a monochromatic beam
Q84: Scandium, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2997/.jpg" alt="Scandium, Sc,