A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state. What is the frequency of vibration of the molecule? (h = 6.626 × 10-34 J ∙ s, 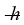 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
Definitions:
German Cartoonist
An artist from Germany who specializes in creating cartoons, which are humorous or satirical sketches and drawings.
Modern Doll
A contemporary toy representing a human figure, often featuring up-to-date fashion, technology, or design elements.
Gender Gap
The disparity between males and females in various aspects of life, including but not limited to income, political representation, employment, and education.
Gender Discrimination
Unjust or prejudicial treatment of individuals based on their gender, affecting opportunities, resources, and rights.
Q1: A laser emits light of wavelength 463
Q1: In the figure, the image is viewed
Q10: One report on death-related practices among American-Indian
Q19: A 440-nm spectral line is produced by
Q19: How fast must a proton move so
Q26: Which of the following is an example
Q28: In the world today, relatively few people
Q34: Dying persons with a terminal illness are
Q45: A microwave oven operates with sinusoidal microwaves
Q79: A convex lens has a focal length