Two deuterium nuclei, 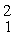 H, fuse to produce a tritium nucleus,
H, fuse to produce a tritium nucleus, 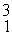
H, and a hydrogen nucleus. A neutral deuterium atom has a mass of 2.014102 u; a neutral tritium atom has a mass of 3.016049 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007276 u. How much energy is released in the process? (1 u = 931.494 MeV/c2)
Definitions:
Doubled
To increase something by 100%, making it twice as large or twice as much in quantity.
Years
Units of time that constitute 365 days (or 366 days in a leap year), commonly used as a measure for time periods in financial calculations.
Annual Growth Rate
The percentage increase in the value of an investment or portfolio over the period of a year.
Price of Gold
The current market value per ounce of pure gold, which fluctuates based on various economic and geopolitical factors.
Q5: When a certain metal is illuminated by
Q9: A certain diatomic molecule emits a photon
Q9: A 360-nm thick oil film floats on
Q10: Which of the following is correct?<br>A) there
Q10: A measurement of an electron's speed is
Q18: An object is placed 100 cm in
Q20: Calculate the ground state energy of a
Q31: In itself, grief at the time of
Q34: Which of the following is not an
Q66: An optical system comprises in turn, from