Consider the fusion reaction 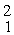
H + 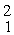
H + 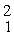
H → 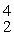
He + 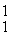
H + 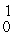
n
The atomic masses are: 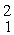
H: 2.01410 u 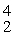
He: 4.00260 u 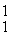
H, 1.00783 u 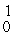
n: 1.008665 u
What mass of deuterium fuel, 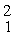
H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Definitions:
Mass Medium
A means of communication that reaches large numbers of people, such as television, radio, newspapers, and the internet.
1950s
The decade spanning from January 1, 1950, to December 31, 1959, often noted for its economic prosperity and emergence of distinct cultural norms in the post-World War II era.
Mass Media
Refers to diversified collections of media technologies that reach a large audience via mass communication.
Impersonal Audience
A broad, undifferentiated group of people to whom communication is directed, without a personal connection or identification.
Q2: College courses on death, dying, and bereavement
Q3: Suppose you were to try to create
Q4: Persons who are dying are most likely
Q6: Death rates<br>A) indicate how long a typical
Q8: Sloane has said that the most remarkable
Q11: In a single-slit diffraction experiment, the width
Q22: In 2009, it is estimated that U.S.
Q24: How many electrons does it take to
Q26: In 2010, the Centers for Disease Control
Q87: Carbon-14 has a half-life of 5730 y.