Short Answer
Consider the fusion reaction 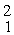
H + 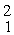
H + 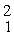
H → 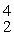
He + 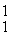
H + 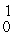
n
The atomic masses are: 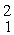
H: 2.01410 u 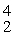
He: 4.00260 u 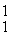
H, 1.00783 u 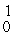
n: 1.008665 u
What mass of deuterium fuel, 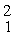
H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Definitions:
Related Questions
Q1: One study of attitudes among Asian and
Q3: The ending of the story of Little
Q9: Our textbook suggests that studying death, dying,
Q13: Changes in American death rates can be
Q14: An electron has a speed of 0.643c.
Q22: The overall, age-adjusted death rate for the
Q23: A situation in which I am concerned
Q25: Death-related attitudes among Asian and Pacific Island
Q30: A diffraction grating has 450 lines per
Q78: A concave spherical mirror with a radius