One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3) . Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3−) and a hydrogen ion (H+) , as noted below. 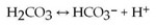 If the pH of blood increases, one would expect ________.
If the pH of blood increases, one would expect ________.
Definitions:
Allergic Reaction
A hypersensitive response of the immune system to substances that are usually harmless to most people, such as pollen, food, or dust.
Antibody Class
Categories of antibodies distinguished by variations in their heavy chain constant regions, affecting their functional location and immune response actions.
IgE
Immunoglobulin E, a class of antibodies that play a crucial role in allergic reactions by recognizing allergens and triggering immune responses.
Immune Response
The body's defense mechanism against foreign substances or pathogens involving both innate and adaptive immune systems.
Q5: You observe the gametes of a fungal
Q6: Which statement is most consistent with the
Q6: Proto-oncogenes _.<br>A) normally suppress tumor growth<br>B) were
Q8: Which of the following is an example
Q21: An atom has four electrons in its
Q25: Why might a point mutation in DNA
Q29: Which of the following can occur by
Q32: Which of the following would you expect
Q45: Gene therapy requires _.<br>A) knowledge and availability
Q68: When comparing the genomes of a bacterial