Listed below are electron dot formulas for several simple molecules and ions.All valence electrons are shown;however,electrical charges have been omitted deliberately. 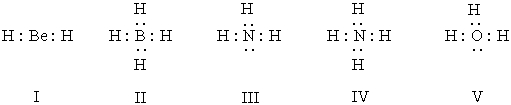 Which of the structures actually bear(s) a positive charge?
Which of the structures actually bear(s) a positive charge?
Definitions:
Hydrogen Bonds
A type of weak chemical bond that is formed when the slightly positive hydrogen atom of one molecule is attracted to the slightly negative atom of another molecule, such as oxygen or nitrogen.
Polar Covalent
A type of chemical bond where electrons are unequally shared between atoms, resulting in partial electrical charges.
Nonpolar Covalent
A type of chemical bond where two atoms share a pair of electrons equally, typically between atoms of similar electronegativity.
Negatively Charged
describes an object or particle that has more electrons than protons, resulting in a net negative electric charge.
Q3: Resistance to change is never appropriate or
Q4: Interviewers should only ask for previously planned
Q9: The thought speed/speech speed differential can be
Q24: Prefabricated groups are so rigidly designed that
Q24: The least stable conformation of 2,3-dimethylpentane,viewed through
Q71: The IR stretching frequency occurs at the
Q79: Which of the following can be described
Q105: Hearing is a natural physiological process,but effective
Q121: Identify the atomic orbitals in the N-O
Q124: Which of the following is true of