Identify the atomic orbital.The lone pair electrons on the B atom are contained in: 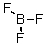
Definitions:
Large Hotel
A significant accommodation facility offering a range of services, often including rooms, food, and entertainment, for travelers and guests.
Flexibility
The ability to adapt to changes or to adjust operations, policies, or procedures as required by circumstances or business needs.
High-Involvement Firms
Organizations that engage employees in decision-making processes, contributing to a sense of ownership and motivation.
Pay for Knowledge
A compensation strategy where employees are paid based on the skills, knowledge, and abilities they bring to their job, rather than solely on their job title or position.
Q4: The development of shared realities and the
Q7: Of the following common organic solvents which
Q17: What is the hybridization of the O
Q18: Which of the following represent a pair
Q33: Defensive organizational climates are characterized by evaluation,control,strategy,neutrality,certainty,and
Q44: Draw a dash-wedge structure for (2R,3S)-2-chloro-3-methylpentane
Q59: Which of the following species contributes more
Q87: Which is a meso compound?<br>A) (2R,3R)-2,3-Dibromobutane<br>B) (2R,3S)-2,3-Dibromopentane<br>C)
Q132: Which compounds above (I-IV)form a set of
Q133: In which structure(s)below does the oxygen have