The reaction, 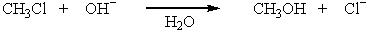 has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
Definitions:
Displaying Wealth
Showing off one's financial assets or luxurious possessions to others as a mark of social status.
Ostracism
The exclusion or rejection of an individual from a social or professional group.
Silent Treatment
A form of psychological manipulation or punishment where communication is withheld from the target as a means of expressing disapproval or to induce feelings of insignificance.
Aggressive Actions
Behaviors intended to cause harm or pain to another person, often as a result of feelings of anger or hostility.
Q5: Which of the following reactions would
Q25: Ambident nucleophiles are ones which can react
Q29: Which proton(s)of the compound below would appear
Q40: The two compounds shown below are: <img
Q42: The most stable conformation of butane is:
Q59: The The molecules shown are: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5901/.jpg"
Q89: The device that is used for measuring
Q93: An alkene adds hydrogen in the presence
Q95: Which would be the major product of
Q149: Draw a dash-wedge structure for (2R,4S)-2,4-dibromo-2-chloropentane