The reaction, 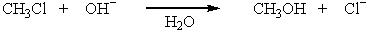 has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
has the following thermodynamic values at 27ºC: H = -75.3 kJ mol-1; S = 54.4 J K-1 mol-1.What is the value of G for this reaction?
Definitions:
Electrical Charge
A fundamental property of matter that exhibits positive or negative qualities and determines the electromagnetic interactions between particles.
Processed Information
Data that has been interpreted, organized, or structured in a meaningful way to support decision-making, understanding, or analysis.
Neuron
A distinct cell that carries messages through the nervous system; identified as a nerve cell.
Parkinson's Disease
A degenerative disorder of the central nervous system that mainly affects the motor system, leading to tremors, rigidity, and slow movement.
Q3: An increase in the temperature at which
Q12: A compound X with the formula C<sub>7</sub>H<sub>10</sub>
Q28: How many <sup>13</sup>C signals would 1,3-dichlorobenzene give?
Q30: Which of the following structures represents bicyclo[3.2.1]octane?
Q42: Which of these molecules is not expected
Q64: What is the molecular formula for the
Q68: In the presence of light,ethane (1 mol)reacts
Q76: A correct name for the following compound
Q88: What is the major product for the
Q101: What is a correct name for the