The thermodynamic parameters at 298 K for the following reaction are given below. 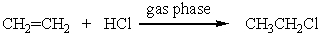 Hº = -64.9 kJ mol-1
Hº = -64.9 kJ mol-1
Sº = -131 J K-1 mol-1
Gº = -25.8 kJ mol-1
Which of the following statements is true of the reaction?
Definitions:
Tax Burden
The impact of taxation on an individual or entity, often expressed as a percentage of income or revenue.
Government Imposes
The act of establishing regulations, taxes, or penalties by the government on businesses or citizens.
Per Unit
A term used to express the cost, price, or quantity concerning a single unit of a product or service.
Tax Burden
The measurement of taxes paid by an individual or business, expressed as a percentage of income or profit.
Q8: If trans-2-butene is treated with meta-chloroperbenzoic acid
Q28: What product is formed when 2,3,3-trimethylhept-1-en-6-yne is
Q43: Complete the following reaction sequence,giving structures for
Q45: What is the major product of the
Q59: Which of the following will have the
Q62: What is the chief product of the
Q64: Which one of the following best represents
Q82: The major product(s)for the following reaction would
Q123: The two compounds shown below are: <img
Q136: Which S<sub>N</sub>2 reaction will occur most