Which of the following structures contribute(s) to the resonance hybrid of the intermediate formed when chlorobenzene undergoes para-chlorination? 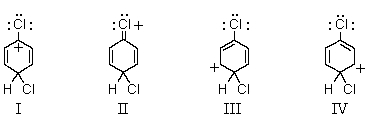
Definitions:
Orbital
A mathematical function describing the location and wave-like behavior of an electron or pair of electrons in an atom.
Electron
A subatomic particle with a negative charge, orbiting the nucleus of an atom and involved in chemical bonds and electricity.
S Orbital
A type of atomic orbital that is spherical in shape, representing the probability distribution of an electron.
Shape
The external form, contours, or outline of an object or figure, often influencing its functionality and perceived aesthetics.
Q52: (Trifluoromethyl)benzene,C<sub>6</sub>H<sub>5</sub>CF<sub>3</sub>,will<br>A) nitrate rapidly in the ortho-para positions.<br>B)
Q75: The major product(s),A,of the following reaction, <img
Q82: Which of the following dieneophiles is most
Q84: Which of the following compounds would you
Q107: Which of these liquids would be unsuitable
Q111: What product would result from the following
Q111: Complete the following reaction sequence,giving structural details
Q113: Estimate the stabilization energy for 1,3-cyclohhexadiene
Q118: What is the product,W,of the following reaction
Q161: Which compound would have a UV absorption