Which of the following compounds would be the weakest base? 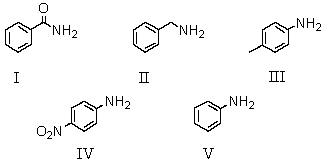
Definitions:
Equilibrium Reaction
A reversible chemical reaction where the rate of the forward reaction equals the rate of the backward reaction, resulting in stable concentrations of reactants and products.
Activation Energy Barrier
The minimum amount of energy required to initiate a chemical reaction.
Collision Theory
A theory that explains how chemical reactions occur and why rates of reactions vary for different reactions.
Forward Reaction
A chemical reaction where reactants convert into products, typically highlighted in a chemistry equilibrium context.
Q11: Amines are known to rapidly undergo pyramidal
Q33: Which compound below does not obey the
Q40: Which of the following is a keto-enol
Q49: Which of these amino acids contains a
Q50: In which of these species are all
Q61: What is the final product of this
Q63: Which of the following hydrogens is the
Q83: Which type of lipid gives these products
Q92: Which of these lipids does not yield
Q97: A compound with an OH and OR