Refer to the following figure when answering
Figure 16.2: Consumption Function 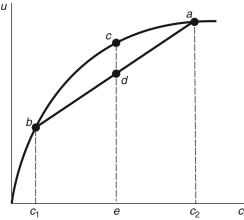
-In Figure 16.2 above, point e is equal to:
Definitions:
Rate Law
An equation that relates the rate of a chemical reaction to the concentration of its reactants, often in the form rate = k[A]^x[B]^y, where k is the rate constant.
Chlorination
A chemical process that introduces chlorine atoms into a molecule.
Acetone
A volatile, colorless liquid used as a solvent and an antiseptic; it's the simplest and most important of the ketones.
ΔH°
The standard enthalpy change of a reaction, indicating the overall heat absorbed or released under standard conditions.
Q7: In the stylized DSGE model in the
Q14: Economists have done a good job of
Q26: The labor market equilibrium determines the market
Q52: In 2012, the United States' trade balance
Q61: Consider Figure 20.3. If the economy initially
Q71: Which of the following goods would you
Q81: Tobin's q is:<br>A) the ratio of stock
Q100: Precautionary savings lead households to act as
Q102: Consider Figure 12.8. You are chairman of
Q103: Since the 1990s, the country with the