Refer to the information provided in Figure 11.7 below to answer the questions that follow.
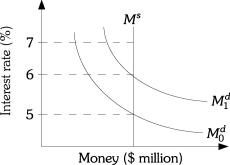 Figure 11.7
Figure 11.7
-Refer to Figure 11.7.If the demand for money curve shifts from to and the interest rate remains at 5%,there will be 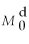
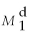
Definitions:
Concentration Effect
The influence on the rate of a chemical reaction of the concentration of reactants; generally, an increase in concentration leads to an increase in reaction rate.
Collision Theory
A theory that proposes chemical reactions occur when reactant molecules collide with sufficient energy and an appropriate orientation.
High Energy
A term used to describe processes, particles, or quantum states having relatively large amounts of kinetic or potential energy.
Particle Motion
The description of the kinetic activity of particles in matter, affected by temperature and physical state, underlying the principles of diffusion and gas behavior.
Q18: Refer to Table 11.1.If it costs $7
Q62: Which of the following events will lead
Q69: Suppose the wage rate in the labor
Q72: During 2013, T Company engaged in the
Q72: Refer to Figure 12.5.Which of the following
Q80: The discount rate cannot be used to
Q84: Investors may wish to hold bonds when
Q95: Of the tools available to the Fed
Q102: Following are the income statement and some
Q111: Electro City,a retailer of electronics,has 2,000 different