Use the following to answer the question: 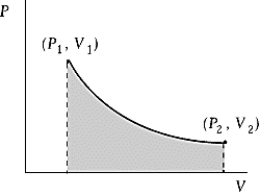
-An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Definitions:
True Lease
A leasing arrangement where the lessor retains substantial risks and rewards of ownership of the leased asset.
Down Payments
Initial payments made when purchasing costly items on credit, representing a fraction of the purchase price.
Lessees
Individuals or entities that obtain the right to use and control a leased asset for a specified period in exchange for payment to the lessor.
Borrow Money
The act of obtaining funds from another party with the promise to return the principal amount along with agreed upon interest.
Q9: A refrigerator extracts 25 kJ from a
Q16: The standing waves in air in
Q22: The electric field for an infinite
Q22: You connect two similar heating coils in
Q38: A noisy workplace has a noise level
Q41: A stretched string of length L,fixed at
Q46: The surface charge density for r
Q78: In your four years at college you
Q83: The molar specific heat of copper
Q89: The pressure of a mass of