Multiple Choice
Use the following to answer the question: 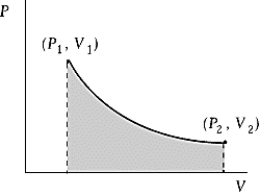
-An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasi-static,isothermal expansion until its pressure is reduced to 50 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Understand how special pricing (such as sales or discounts) affects consumer surplus.
Interpret demand curves and calculate consumer surplus from graphical representations.
Demonstrate how utility theory applies to real-world decisions and consumption patterns.
Understand the relationship between marginal utility, total utility, and the decision-making process in consuming additional units of a good or service.
Definitions:
Related Questions
Q1: The current in a wire varies
Q9: An oil droplet of mass 1.00
Q25: A uniform line charge of linear
Q28: An ideal monatomic gas has a molar
Q29: If the absolute temperature of an object
Q42: An electron is released from rest
Q47: The mass on the end of the
Q51: A device used to measure the
Q53: If two elements of a circuit are
Q101: Geologists use explosives to map the subterranean.The