Use the following to answer the question: 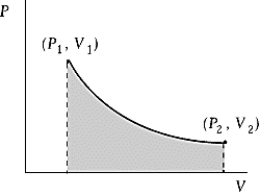
-An ideal gas initially at 50ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Definitions:
Alcohol Tax
A special tax imposed on alcoholic beverages to generate revenue and/or discourage excessive consumption owing to health or social reasons.
Compliance Costs
Expenses incurred by businesses or individuals to adhere to laws, regulations, and standards.
U.S. Income Tax
A tax imposed by the U.S. government on the income earned by individuals, corporations, estates, and other entities.
Vertical Equity
The principle that people with higher incomes should pay more in taxes than those with lower incomes, reflecting a progressive tax system.
Q4: You want to use a metal bar
Q5: A refrigerator extracts heat Q from a
Q17: The electric potential in a region of
Q30: Mountaineers say that you cannot hardboil an
Q36: The coefficient of thermal expansion of
Q51: A vibrating tuning fork of frequency 1080
Q53: The angle of the shock wave produced
Q56: The energy of a simple harmonic oscillator
Q80: Calculate the change in electrostatic potential energy
Q111: A physical pendulum oscillates with small amplitude