Use the following to answer the question: 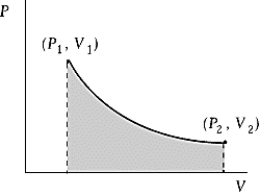
-An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Definitions:
Corn
A cereal plant that yields large kernels set in rows on a cob; an important crop for food, animal feed, and industrial products.
Bushel
A unit of volume used primarily in the United States for measuring agricultural commodities, equivalent to approximately 35.24 liters.
Marginal Costs
The additional cost incurred in producing one more unit of a good or service, critical in decision-making regarding production volumes.
Standby Seats
Airline or event tickets made available last minute, typically at a lower price, for customers willing to wait for no-shows or extra capacity.
Q2: The electron volt is a unit of<br>A)capacitance<br>B)charge<br>C)energy<br>D)momentum<br>E)potential
Q3: What is the phase difference at any
Q9: Dielectric breakdown occurs in the air at
Q33: Which of the following statements is not
Q39: A cylinder contains 20 L of air
Q45: An electron (q =+e)and a positron (q
Q52: The equation that gives the particle
Q55: Suppose the apparent wavelength of the known
Q58: An ideal gas initially at 100ºC and
Q63: In an adiabatic reversible compression of an