Use the following to answer the question: 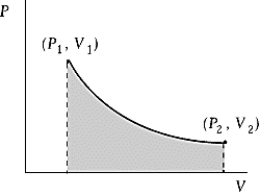
-An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
Definitions:
Tax On Gasoline
A government-imposed charge on the sale of gasoline, typically used to fund transportation infrastructure or environmental projects.
Technology Spillovers
The process through which innovations and technologies developed in one sector or firm spread to other sectors or firms.
Patent Protection
The granting of a legal right to an inventor to exclude others from making, using, or selling their invention for a limited period.
External Benefit
A benefit that an action or activity provides to individuals or groups who are not directly involved in it.
Q10: An infinitely long cylindrical shell of
Q14: A piano wire has a tension
Q18: You connect three capacitors as shown
Q41: A thermometer is constructed by filling
Q51: The graph shows the average power delivered
Q56: The energy of a simple harmonic oscillator
Q72: A state variable is one that allows
Q77: A vibrating tuning fork of 725
Q83: The molar specific heat of copper
Q99: In a system composed of an ideal