Use the following diagram to answer the next problem. 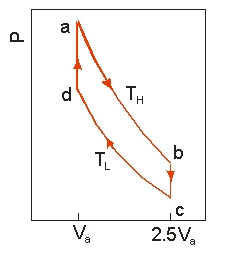 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much work is obtained from the engine in each cycle?
Definitions:
Hierarchical
characterizes systems or organizations structured in levels of rank or authority, where higher levels have control over the lower ones.
Approach-Inhibition Model
A psychological theory suggesting that power increases an individual's tendency to approach positive stimuli and outcomes, while reducing their perception of risks.
Teachers
Educators or instructors who facilitate learning for students, typically in a school, college, or university setting.
Students
Individuals enrolled in an educational institution for the purpose of learning.
Q18: An equation that gives the particle displacement
Q23: If a mass of oxygen gas occupies
Q27: The frequency spectrum of the composite wave
Q28: An ideal monatomic gas has a molar
Q32: An electron is accelerated from rest
Q52: If the engine operates at 50 cycles
Q65: A room measures 3 m
Q66: If it takes you 5 minutes to
Q77: The equation of a body in
Q83: The molar specific heat of copper