Use the following diagram to answer the next problem. 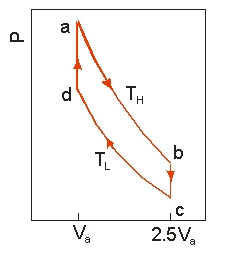 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much heat is absorbed in going from a b?
Definitions:
Acidic Substance
A substance with a pH less than 7, which donates hydrogen ions when dissolved in water.
Basic Substance
A fundamental material or compound that is essential for a particular biological function or structure; often refers to simple, key molecules in biochemistry.
Amino Acids
Organic compounds that serve as the building blocks for proteins, essential to numerous processes in living organisms.
Triglycerides
A type of fat (lipid) found in your blood, made up of a glycerol molecule attached to three fatty acids, used by the body for energy or stored as fat.
Q1: If a steam engine operates at half
Q3: A heat engine absorbs 150 J of
Q18: At liquid <sup>4</sup>He temperatures (< 4.2 K),the
Q24: While you are standing on a corner,a
Q28: A sphere of radius 8.0 cm
Q41: Which of the following statements about electromagnetic
Q43: If you were to reduce the amplitude
Q70: Which of these five terms is most
Q70: An 8.0-kg block is attached to a
Q72: A violinist is tuning the A string