Use the following figure for the next problem. 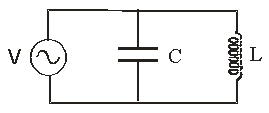
An AC power supply, V = 20 V sin 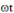 where
where  = 200 rad/s is connect to an inductor L = 180 mH and a capacitor C = 25
= 200 rad/s is connect to an inductor L = 180 mH and a capacitor C = 25 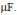 XL = 36 ohms, XC = 200 ohms, Z = 43.9 ohms
XL = 36 ohms, XC = 200 ohms, Z = 43.9 ohms
-What is the peak current flow out of the AC power supply?
Definitions:
Ions
Atoms or molecules that have gained or lost one or more electrons, thus acquiring a charge.
Acid
A substance that is a hydrogen ion (proton) donor; acids unite with bases to form salts. Compare with base.
Atomic Mass
The mass of an atom expressed in atomic mass units (amu), essentially equivalent to the number of protons and neutrons in the nucleus.
Atom
The basic unit of a chemical element, consisting of a nucleus of protons and neutrons, with electrons orbiting the nucleus.
Q2: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6081/.jpg" alt=" A positively charged
Q10: A coil with a self-inductance of 6.5
Q19: Which of the following experiment(s) illustrates the
Q31: A series RLC circuit with L
Q33: A 60-W light bulb emits spherical electromagnetic
Q33: The principle of superposition states:<br>A) The effect
Q34: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6081/.jpg" alt=" A
Q45: A long solenoid of radius 3 cm
Q59: The minimum angle of deviation produced by
Q66: Lea charges a capacitor and then discharges