 Figure 1
Figure 1
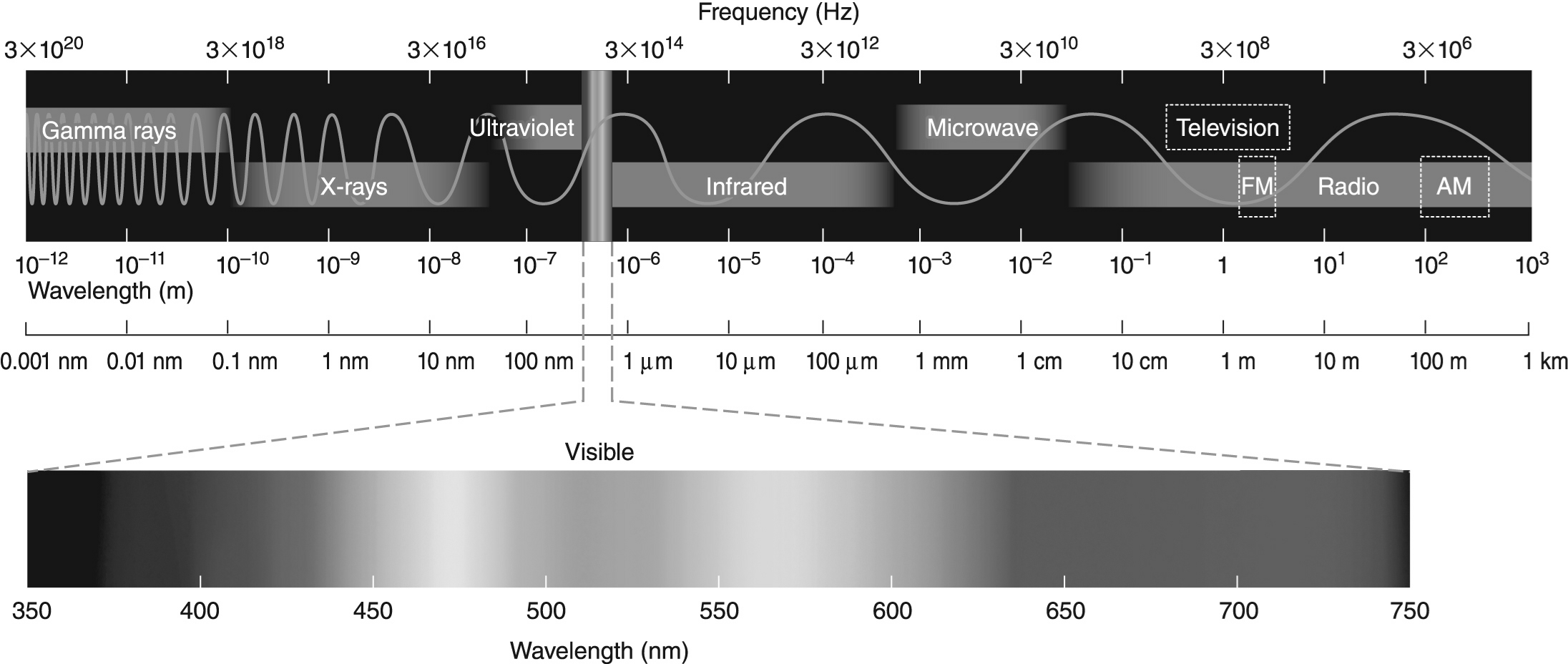
-The difference in energy between the n = 2 and n = 1 electronic energy levels in the hydrogen atom is 1.6 * 10-18 J. If an electron moves from the n = 1 level to the n = 2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown above to answer this question.
Definitions:
ROM
Read-Only Memory, a type of storage medium that is used to store firmwares that are seldom changed during the life of the system.
RAM
Volatile memory in computing that is used to store data temporarily while a computer is running, facilitating quick access by the processor.
Libraries
Collections of resources or data in computing, used for storing files, documents, or code libraries in organized manners for easy access and management.
Active Window
The currently focused window on a computer screen, which receives the input from the keyboard and mouse.
Q6: Which of the following statements is TRUE
Q21: In Figure 2, at which position must
Q28: Bronfenbrenner terms the culture in which an
Q28: An unbound orbit results when a satellite
Q31: On average, adults remarry within _ year(s)
Q38: In terms of frames of reference, explain
Q45: The average red giant in the night
Q66: Harold knows he will not live much
Q69: Which of the following statements is NOT
Q91: Briefly describe the process of mourning in