The 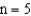 electronic energy level in a hydrogen atom is
electronic energy level in a hydrogen atom is 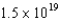 J higher than the
J higher than the 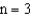 level.If an electron moves from the
level.If an electron moves from the 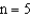 level to the
level to the 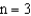 level then a photon of wavelength
level then a photon of wavelength
Definitions:
Contingent Liability
A potential obligation that may arise in the future depending on the occurrence of a certain event.
Financial Statement
A formal record of the financial activities and position of a business, person, or other entity.
Numerical Probability
A quantifiable measure that expresses the likelihood of occurrence of an event, presented as a number between 0 and 1.
Gross Earnings
Gross Earnings are the total amount earned by an individual or entity before any deductions such as taxes, benefits, and other payroll deductions.
Q5: An asteroid with an albedo of 0.1
Q7: According to Erikson,the focus of adolescence is
Q26: Our Solar System is likely unique among
Q32: All planets orbit the Sun in the
Q44: The cosmological principle states that<br>A) the universe
Q44: Which of the following is NOT considered
Q61: Permanent magnetism in the Earth's solid iron
Q66: Rings that project to look like they
Q67: How much fainter are the faintest stars
Q100: Research suggests that _ adolescents display classic